EU regulatory landscape for medicinal products
The European Union is not a Federation, but a Community of States
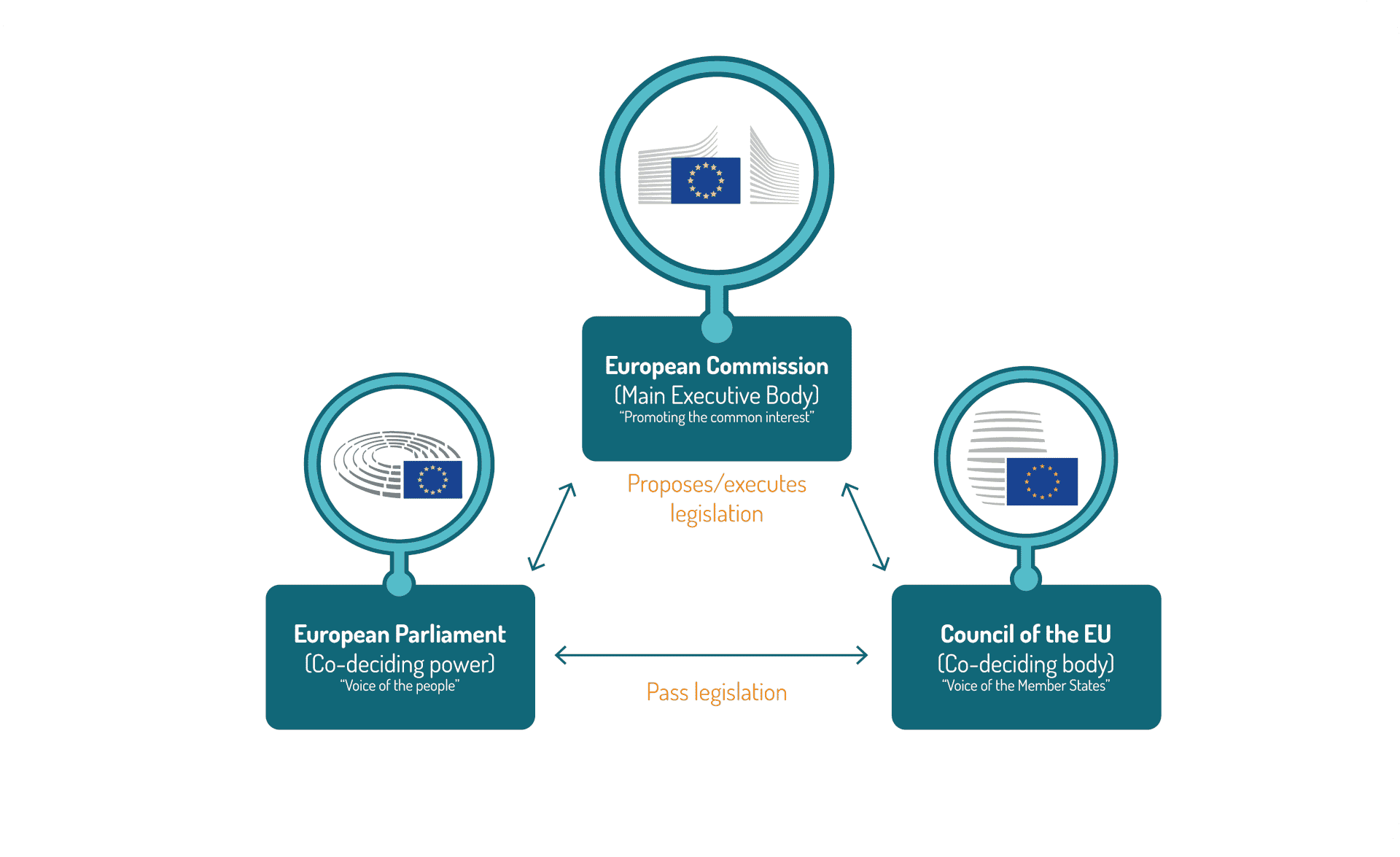
The European Commission (EC) is the executive body of the EU, in charge of proposing new legislation to the European Parliament and the Council of the European Union as well as implementing new legislative acts once co-approved by European Parliament and the Council of the European Union.

EU Legislative & Regulatory Bodies
In certain areas, the EU can only support, coordinate or complement the action of Member states. It has no power to pass laws and may not interfere with member countries’ ability to do so. In these areas, the EU has what the treaties refer to as ‘supporting competences’. It is notably the case for public health. At the EU level, the complexity of the European Union is partly due to the multiple bodies involved as well as a variety of procedures for medicinal products’ registration, as member states have kept the competency for decisions on the regulatory status of products (also, clinical trial authorizations took place at the national level for quite some time before). Competent authorities therefore exist within each Member state, in addition to the European Medicines Agency (EMA).
The following bodies are respectively involved in the shaping of the EU legislative and regulatory landscape for medicinal products.
The PRI collaborates with various stakeholders at the national and European level.
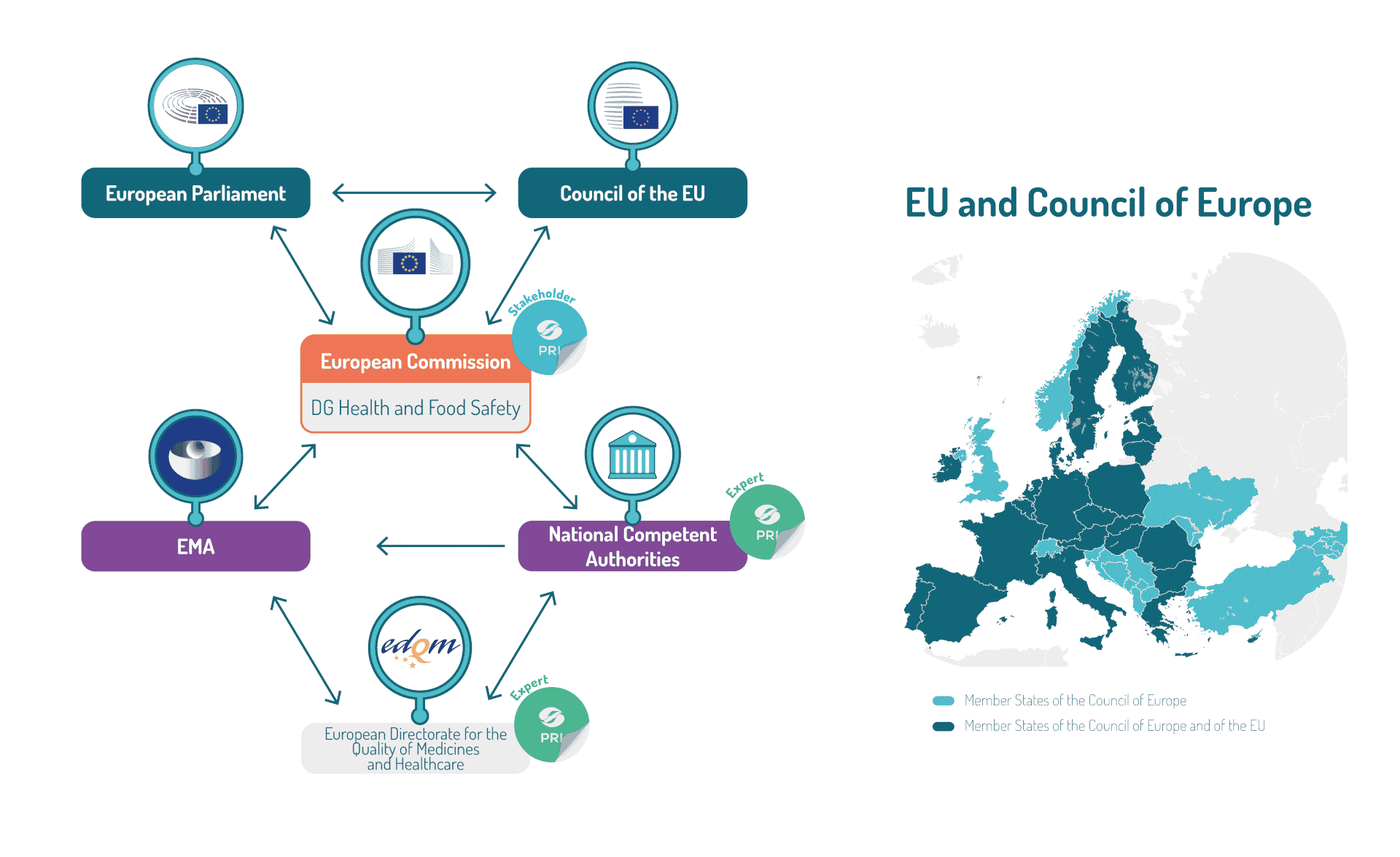
As perfectly explained here, the system for regulating medicines in Europe is unique. It is based on a closely-coordinated regulatory network of national competent authorities in the Member States of the European Economic Area (EEA), working together with the European Medicines Agency (EMA) and the European Commission. EMA oversees the scientific evaluation of the products while the European Commission grants the centralized marketing authorizations when the products are registered through the centralized procedure. EMA operates at the heart of the European medicines regulatory network to allow for collaboration and coordination between the national competent authorities for veterinary and human medicines. Such national competent authorities are also the suppliers of the thousands of experts involved within the EMA committees.
Additionally, the EU being a member of the Council of Europe, Member states are also represented within the European Directorate on Quality of Medicines & HealthCare (EDQM). The EDQM is composed of nine entities, one of them being the European Pharmacopoeia Department (EPD). This department is responsible for preparing the texts of the European Pharmacopoeia.
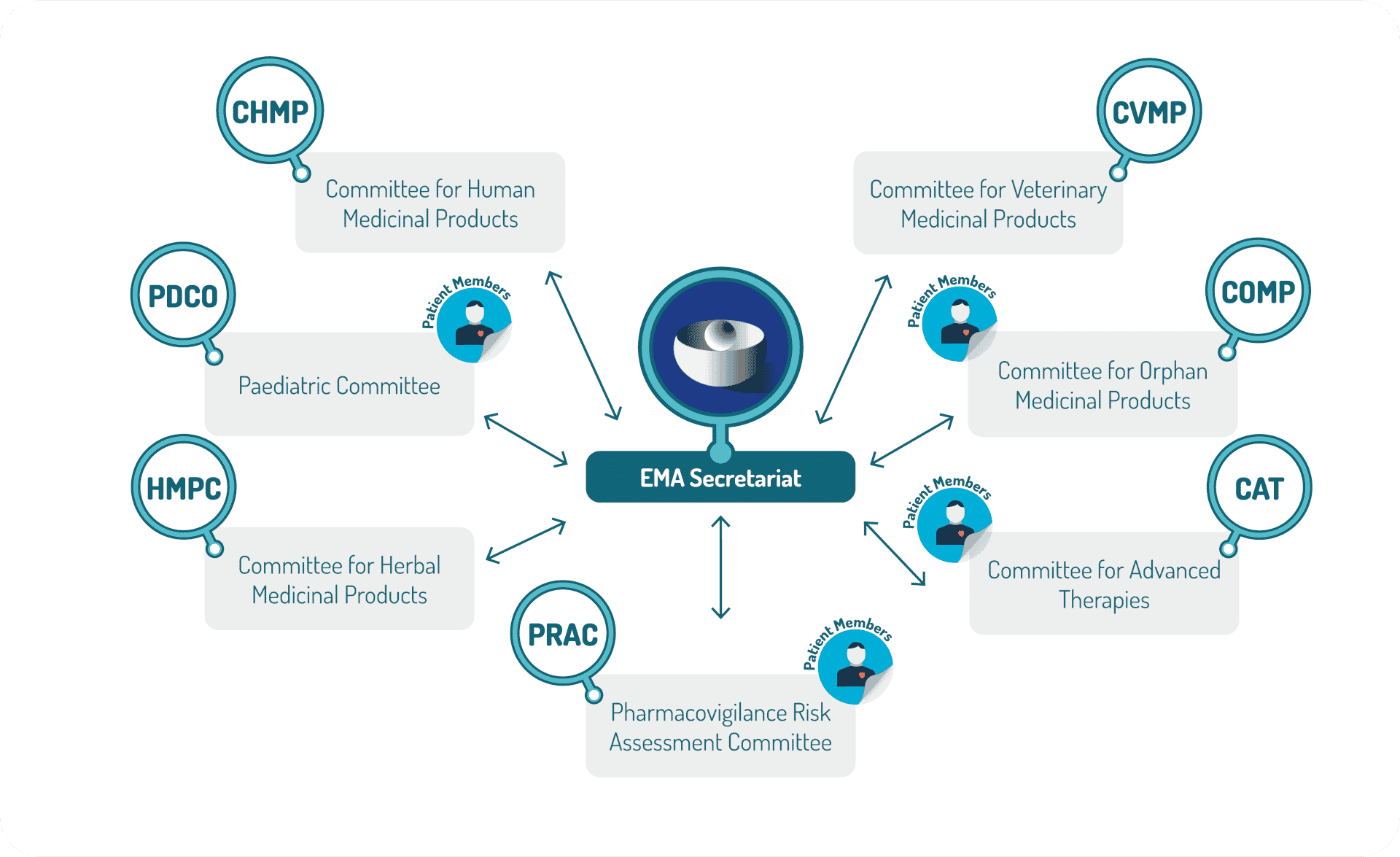
EU competent authority involved in scientific assessment
EMA is organized into 7 committees and Co-ordination groups within which a number of working parties are established. The EMA committees contain members nominated by the national competent authorities.
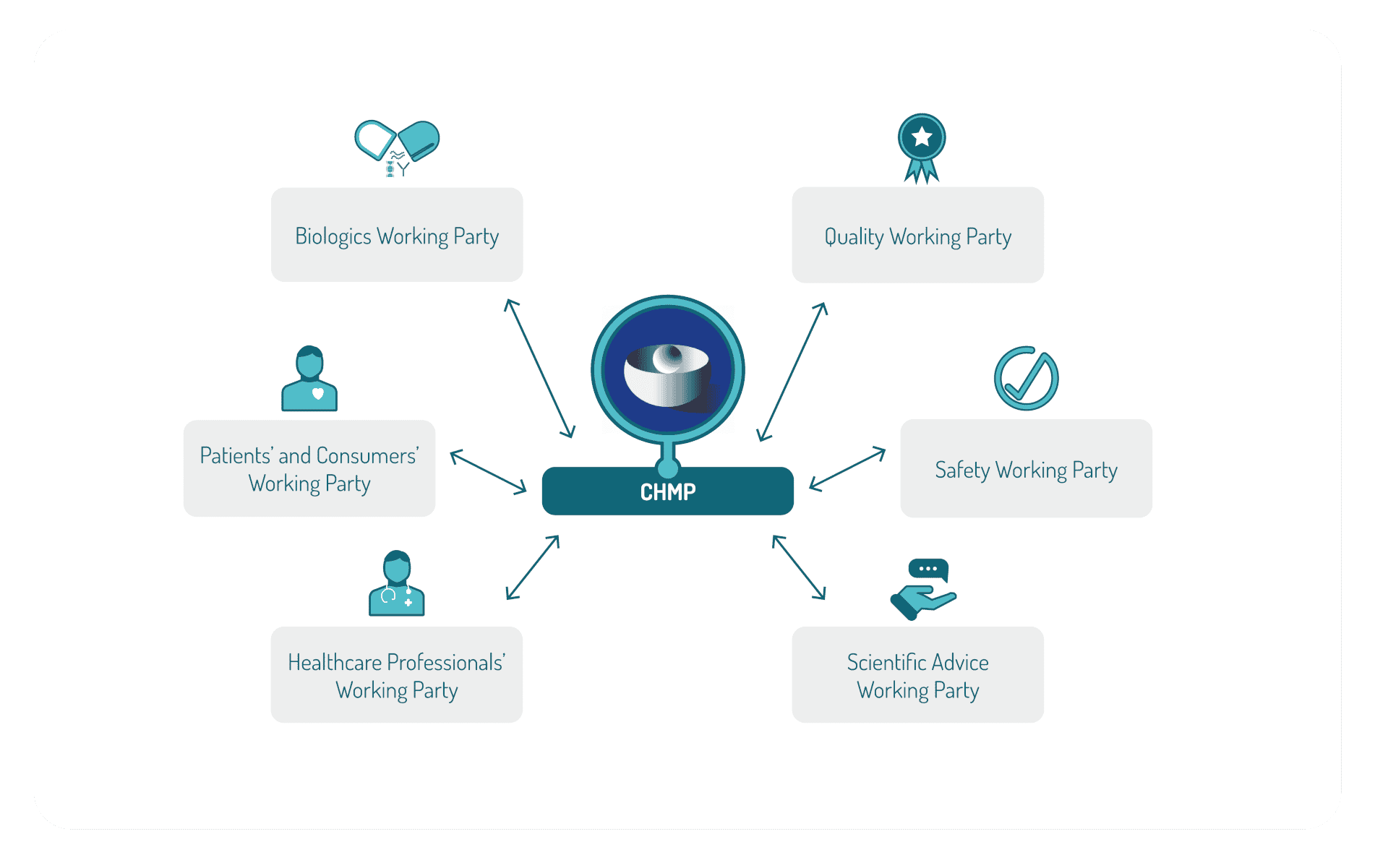
The CHMP committee (Committee for Medicinal Product for Human Use) is responsible for the scientific evaluation for product approval and has established several working parties, such as: Biologics Working Party, Quality Working Party, Safety Working Party, the Scientific Advice Working Party, Healthcare Professionals’ Working Party, Patients’ and Consumers’ Working Party.
At the national level, each Member state has its national competent authority. The Heads of Medicines Agencies (HMA) is the network of the heads of the National Competent Authorities (NCA) whose organisations are responsible for the regulation of medicinal products for human and veterinary use in the Members States and the countries of the European Economic Area (EEA). As one of its many activities, the HMA co-ordinates the mutual recognition (MRP) and decentralised procedures (DCP).
Marketing Authorization
Considering a European or national procedure
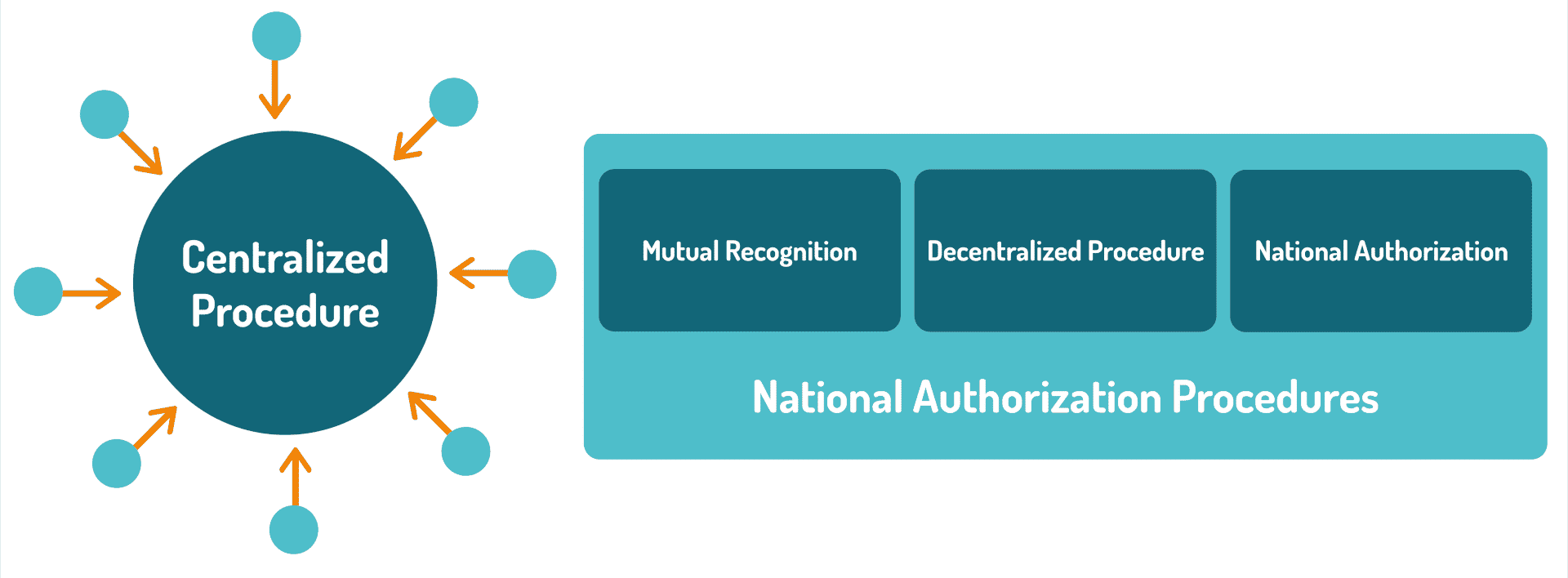
In the European Union, there are two ways to obtain a medicinal product marketing authorization: the centralized procedure and the national authorization procedures.
The centralized procedure associated with scientific evaluation for product approval at the EMA level is compulsory for:
- human medicines containing a new active substance to treat: human immunodeficiency virus (HIV) or acquired immune deficiency syndrome (AIDS); cancer; diabetes; neurodegenerative diseases; auto-immune and other immune dysfunctions; viral diseases.
- medicines derived from biotechnology processes, such as genetic engineering;
- advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines;
- orphan medicines (medicines for rare diseases);
- veterinary medicines for use as growth or yield enhancers.
On the other hand, it is optional for other medicines:
- containing new active substances for indications other than those stated above;
- which represent a significant therapeutic, scientific or technical innovation;
- whose authorisation would be in the interest of public or animal health at the EU level.
In this last situation, a decentralised procedure and a mutual recognition procedure are in place in order to obtain marketing authorization in a handful of EU Member states. If there is disagreement between Member States during the assessment of the submitted data, the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh) considers the matter and strives to reach an agreement. If this is not possible, the Member State responsible for the product brings the case to the attention of the EMA Committee for Medicinal Products for Human Use (CHMP) for arbitration.
Scientific evaluation of microbiome-based medicinal products
Pertaining to microbiome-based medicinal products for human use, a few EMA committees could be involved, such as the CHMP, but also the CAT (Committee for Advanced Therapies) in the case of products developed for gene therapy, or the PCDO (Paediatric Committee) in charge of the Pediatric Investigation Plan approval. In the case of orphan diseases, the Committee for Orphan Medicinal Products (COMP) will also be involved in recommending orphan designation of medicines for rare diseases.
As microbiome-based medicinal products can be developed in various indications and represent various technologies, while being biological medicinal products by nature, their assessment may require the participation of various committees and working parties for a single product.
The lack of expertise in “transversal microbiome science” within the EU and national authorities, as well as the lengthy procedures and the complexity of the system have been identified by the industry as a major issue at the EU level. Nevertheless, knowledge is improving across the EU and recent calls for proposals organized by the European Commission are focusing on improving microbiome regulatory science at the EU level with the intention of accelerating translation from academia to industry as well as improving regulators’ involvement in projects financed under the Horizon Europe framework program. Such positive developments is exemplified by the various partnerships in which the PRI is actively involved. Indeed, pre-competitive regulatory science projects involving regulators from EU and national competent authorities would be highly beneficial for EU attractiveness in the domain as it could improve microbiome science knowledge within competent authorities and allow the industry to engage in more robust science-driven developments while improving time to market and patient access.
EU moving towards stronger regulatory harmonisation
Today, PRI sees a trend towards stronger harmonisation particularly for highly innovative products, such as ATMP. More recently, the new regulation on clinical trials is moving us towards a centralised procedure for clinical trials authorisation applications. On the veterinary side, the new regulation also brings the highest level of harmonisation after years of operations under a Directive. Overall, the EU is strengthening harmonisation in order to improve regulatory attractiveness and acceleration of patients’ access to innovative medicinal products in general.
Nevertheless, in the case of microbiome-based medicinal products, discrepancies still exist, such as for example decisions on the regulatory status for fæcal material, in the context of FMT (classified as a medicinal product by some Member states, as Tissue and Cells in others, or with no particular status in others still), or the variability of the assessment between national competent authorities due to the heterogenous knowledge on microbiome science. The current revision of the EU general pharmaceutical legislation will likely be an important opportunity to improve harmonisation.
By promoting the integration of microbiome regulatory science at the EU and national levels, the PRI hopes to play an active role in the improvement and harmonisation of microbiome-based medicinal products scientific assessment in Europe.