EU Microbiome
Regulatory Science Center
Applying regulatory science for new therapies and diagnostics
The PRI is the European Microbiome Regulatory Science Expertise Center, created in 2010 to support the development and registration of therapeutic and diagnostic products emerging from microbiome science.
We work to educate and contribute to the conditions of success for future medicinal innovations emerging from microbiome science, and our ultimate objective is to see these therapeutic innovations achieve EU marketing authorization, thereby giving European patients access to registered microbiome-based medicinal products or validated diagnostics.
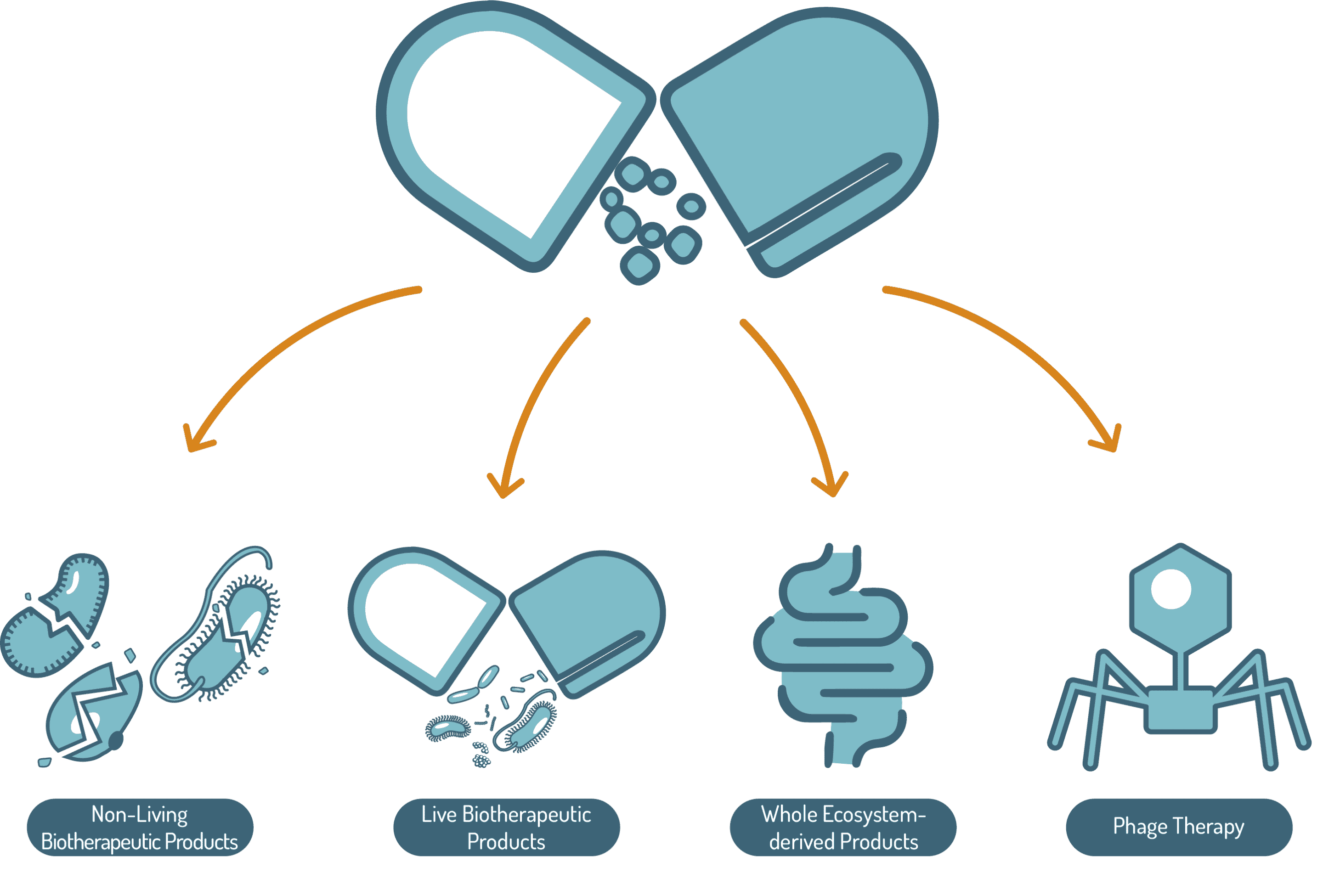
Microbiome-based medicinal products
Complex technologies with ecosystemic effects
The future of human and veterinary medicine has been forever changed by the fundamental disruption of microbiome science – we are only now understanding ‘health’ as an ultimate result of a complex interaction between host and one’s microbiome.

The EU Regulatory Framework & Landscape
As for any other medicinal products, microbiome-based medicinal products must comply with the pharmaceutical standards for quality, safety and efficacy and will require a medicinal product marketing authorization to reach market.
The system for regulating medicines in Europe is unique. It is based on a closely coordinated regulatory network of national competent authorities in the Member States of the European Economic Area (EEA), working together with the European Medicines Agency (EMA) and the European Commission.
Overall, most microbiome-based medicinal products are moving through the development process based on interactions between developers and regulators at the national level in a select few of EU member states.

Microbiome Regulatory Science News & Events
2024 Pharmabiotics Conference & Partnering – Lille
Last month over 160 attendees joined together in Lille, France for the 9th annual Pharmabiotics Conference & Partnering event organized by PRI and I-Squared Communications. The 2024 edition focused on the theme of ‘An Evolving Regulatory Landscape for...
Céline Druart appointed PRI Executive Director following Magali Cordaillat-Simmons’ decision to step down
Effective from 24 January 2024, Céline Druart has been appointed and will assume the role of PRI's new Executive Director. After 13 years with the PRI, Dr. Magali Cordaillat-Simmons will resign from her position as PRI's Executive Director and pursue other...
Pharmabiotics Conference 2024 – “An Evolving Regulatory Landscape”
The Pharmabiotics Conference Advisory Committee with the PRI Team and I-Squared Media are pleased to announce the upcoming 9th edition of the annual Pharmabiotics Conference & Partnering Event, 12-14 March, in Lille. This year’s theme, ‘An Evolving Regulatory...